The Effect of CYA on Water Balance and Plaster
The Effect of CYA on Water Balance and Plaster
When you measure total alkalinity in a pool, you are measuring the alkalinity from both carbonate and cyanurate in the water. Cyanuric acid helps to buffer pH in pool water, but it does not protect plaster. Carbonate alkalinity is needed to protect plaster. Therefore, when the Langelier Saturation Index (LSI) is calculated, only the carbonate alkalinity should be used to determine if the water is scaling or corrosive. The following equation should be used to calculate the carbonate alkalinity:
Carbonate alkalinity = total alkalinity – (cyanuric acid concentration/3)
For instance, if the total alkalinity is 80 ppm and the cyanuric acid concentration is 100 ppm,
Carbonate alkalinity = 80 – (100/3)
= 80 – 33
= 47 ppm
= 80 – 33
= 47 ppm
The importance of performing this correction is clear in this example, where a value of 80 ppm might lead you to think that the water was not corrosive, but with a carbonate alkalinity of 47 ppm, the water is clearly on the corrosive side.
Pool testing with plaster coupons was conducted with cyanuric acid levels of 0, 25-50, 110-125 and 200-250 ppm. The water parameters were maintained at pH 7.2 to 7.6, carbonate alkalinity 80-120 ppm, calcium hardness 180-250 and available chlorine 1-4 ppm. After 12 months of operation, the pictures below show surface degradation on the plaster coupons in all pools. Swimming pools maintained with no cyanuric acid stabilizer showed the least surface damage.
Despite the balanced pool water conditions in the pool tests, plaster degradation was still seen. These results indicate that the presence of cyanuric acid in water etches plaster surfaces, and that a minimum amount should be used only if chlorine stabilization is necessary.

Water Balance
pH is the negative log of the hydrogen ion concentration. The following equation expresses the relationship between hydrogenions (H+) and pH:
pH = -log[H+]
where [H+] is the concentration of hydrogen ions.
The negative sign in the equation means that if the level of hydrogen ions goes up, the pH goes down. Conversely, if the level of hydrogen ions goes down, the pH goes up.
The “log” means that drastic changes in [H+] will only make small changes in pH. Conversely, small changes in pH may mean there are drastic changes in [H+]. Following is a short table showing some log values:
This means that if the pH goes down by 1 unit, then the H+ concentration has increased 10-fold.
Chemicals that contribute hydrogen ions to the water are called acids, and they lower the pH. An example is muriatic acid (HCl), also called hydrochloric acid. When HCl is added to water, the following happens:
From this equation, it is clear that muriatic acid is contributing hydrogen ions to the water, and therefore, adding muriatic acid to a pool will lower the pH.
In order to stabilize H+ concentrations in pool water, and therefore stabilize the pH, we use buffers. Buffers are chemicals that can take up, or contribute, H+ as the need arises.
The most common buffer used in pools is carbonate. Carbonate can be present in the water as carbonic acid (H2CO3), bicarbonate (HCO3-), or carbonate (CO32-). The following graph shows how much of each species is present with varying pHi. The pH range for pools (7.2 – 7.8) is highlighted in yellow.
At pool pH values most of the carbonate is present as bicarbonate with some carbonic acid. If an acid is added to the pool, the bicarbonate in the water can take up the hydrogen ions and make carbonic acid:
HCO3- + H+ →H2CO3
So the pH would not go down significantly until you have used up most of the bicarbonate in the water.
Likewise, if you add something with a high pH to pool water that takes away hydrogen ions, carbonic acid can give up a hydrogen ion to replace the one that was lost:
H2CO3 → HCO3- + H+
So the pH would not go up significantly until you have used up most of the carbonic acid in the water.
Cyanuric acid is also a buffer in pool water. The following structure shows that it has the capability to bind and release three hydrogen ions:
When all three hydrogen atoms are attached to the ring, it is called cyanuric acid. The general word “cyanurate” is used for the ring with 1, 2, or all 3 of the hydrogen atoms missing. The following graph shows how much of each species is present with varying pH.
Cyanuric acid is good at buffering pH, but it is not good at protecting plaster. In fact, there is evidence that it can damage plaster. This topic is discussed below. Therefore, when maintaining water balance for a pool or spa, it is important to maintain the proper carbonate alkalinity concentration. The recommended range from the Association of Pool and Spa Professionals (APSP) and the Model Aquatic Health Code (MAHC) is 60-180 ppm.
When a total alkalinity test is performed, it measures all the buffers in the water, including both carbonate and cyanurate. In order to convert a total alkalinity reading to carbonate alkalinity a cyanuric acid conversion factor must be used. The factor is about 1/3, but varies with pH.
The following general equation can be used to calculate the carbonate alkalinity:
Carbonate alkalinity = total alkalinity – (cyanuric acid concentration/3)
For instance, if the total alkalinity is 80 ppm and the cyanuric acid concentration is 100 ppm,
Carbonate alkalinity = 80 – (100/3)
= 80 – 33
= 47 ppm
= 80 – 33
= 47 ppm
The importance of performing this correction is clear in this example, where a value of 80 ppm might lead you to think that the water was not corrosive, but with a carbonate alkalinity of 47 ppm, the water is clearly on the corrosive side.
The value of the cyanuric acid correction factor varies with pH. If a more exact number is needed, the following table should be used.
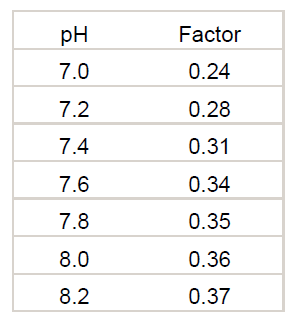
Where carbonate alkalinity = total alkalinity – (cyanuric acid concentration x Factor)
The Langelier Saturation Index (LSI) is often used to predict whether water in a pool will be corrosive or scaling. The factors that go into the LSI calculation are pH, carbonate
alkalinity, calcium hardness, total dissolved solids (TDS) and temperature. Following are the factors from APSP-11.
These factors are used in the following equation: LSI = pH + TF + AF + CF – TDS Factor
The recommended range from APSP-11 is -0.3 to +0.5. Values below -0.3 indicate that the water will be corrosive. Values above +0.5 indicate that the water will be scaling.
The recommended range from APSP-11 is -0.3 to +0.5. Values below -0.3 indicate that the water will be corrosive. Values above +0.5 indicate that the water will be scaling.
It is important that the carbonate alkalinity be used in calculating the LSI, not the total alkalinity.
Plaster
Plaster chemistry is very complex, but the primary components of plaster are Portland cement and aggregatevi. Aggregate is the marble dust, sand, or pebbles used in plaster. Portland cement is used to cement the aggregate particles together into a solid durable surface. When a pool is plastered, the Portland cement undergoes hydration reactions that produce calcium hydroxidevii. Over time, as carbon dioxide migrates through the plaster, the calcium hydroxide is converted into less soluble calcium carbonate. So calcium carbonate is present in the cement holding the aggregate together, and it can also be present in the aggregate itself, particularly if marble dust is used. Marble is typically composed of calcite (CaCO3) or dolomite (CaMg(CO3)2).
The solubility of calcium carbonate is governed by the pH and carbonate concentration of the waterviii, as well as the other factors in the Langelier saturation index calculation. If there is not sufficient carbonate in the water, then calcium carbonate in the Portland cement and aggregate will dissolve into the water, causing corrosion of the plaster surface.
When trichloroisocyanuric acid (trichlor) is used as a sanitizer in a pool, it can affect plaster in a couple different ways. One way is very well understood, and the other way is just now being explored.
The first way that trichlor can destroy plaster is through the low pH of the product. The pH of trichlor is about 3ix. By lowering the pH of the pool, trichlor can lower the carbonate alkalinity concentration, and subsequently lower the LSI. With a lower LSI, plaster degradation is more likely as discussed already
The second way that trichlor can destroy plaster has been seen in a series of studies performed by Arch Chemicals.
In 2004, Arch Chemicals, Inc. (now Lonza) ran laboratory studies to determine the effect of cyanuric acid on sections of white pool plasterx. The levels of cyanuric acid tested were 200 and 500 ppm. After five weeks the cyanuric acid in the water with the plaster coupons had dropped considerably, and surface analysis showed accumulation of cyanuric acid on the plaster in both 200 and 500 ppm. The reaction of cyanuric acid was much faster in the 500 ppm sample as shown in the following graph:
The study also demonstrated that cyanuric acid reacted with components of white plaster, particularly with calcium oxide from the Portland cement, to most likely form calcium cyanurate on the surface of the plaster. This reaction removes the calcium oxide, leaving an etched or disintegrated surface on the plaster.
Based on these initial results, a 6 month tank test was conducted to better understand the effect of cyanuric acid on plasterxi. These sections of pool plaster were 9” by 12” coupons, approximately ½” thick, made in the laboratory using formulations provided by the National Plasterer’s Council. The water in the tank tests was adjusted daily to maintain pH 7.2 to 7.8 and alkalinity 60 to 100 ppm. Because the plaster coupons were new, the pH rose continuously and therefore needed to be adjusted daily. The free available chlorine was maintained at 1 to 4 ppm. Cyanuric acid levels of 0, 25, 50, 100, 250 and 500 ppm were tested. Images taken with a scanning electron microscope at 250 times magnification showed increasing degradation of the plaster surface as the level of cyanuric acid increased (see pictures below).
Picture
Pool testing with plaster coupons was started in May of 2005xii. Pools were run with cyanuric acid levels of 0, 25-50, 110-125 and 200-250 ppm. The water parameters were maintained at pH 7.2 to 7.6, carbonate alkalinity 80-120 ppm, calcium hardness 180-250 and available chlorine 1-4 ppm. After 12 months of operation, the pictures below (14x magnification) show surface degradation on the plaster coupons in all pools. Swimming pools maintained with no cyanuric acid stabilizer showed the least surface damage.
In the tank studies described above, the pH and alkalinity of the tanks ran on the high side (pH ~8, TA ~90 ppm) despite daily adjustments, while the pools were maintained in a balanced condition. Despite the high pH and alkalinity in the tank test, and balanced pool water conditions in the pool test, increased plaster degradation was still seen with cyanuric acid. These results indicate that the presence of cyanuric acid in water etches plaster surfaces, and that a minimum amount should be used only if chlorine stabilization is necessary.
References
i Equilibrium constants from Zumdahl, S.S., Chemistry, D.C. Health and Company, Lexington MA, 1986, Ka(H2CO3) = 4.3×10-7, Ka(HCO3-) = 5.6×10-11.
ii O’Brien, J. E. Morris, J. C., Butler, J. N., Equilibria in aqueous solutions of chloriinternanated isocyanurates, In: Chemical Water Supply Treatment, Chapter 14, pp. 333- 358; edited by A. J. Rubin, Ann Arbor Sciences, Ann Arbor, MI 1974. Presented at the District Symposium, Philadelphia, 1973.
iii American National Standard for Water Quality in Public Pools and Spas, ANSI/APSP-11, 2009, The Association of Pool and Spa Professionals.
iv Model Aquatic Health Code, 2016 Edition, Centers for Disease Control and Prevention.
v Wojtowicz, J.A., Swimming pool water balance, Part 1: The effect of cyanuric acid and other interferences on carbonate alkalinity measurement, JSPSI 1995 1(1), 7-13.
vi Technical Manual, 7th edition, National Plasterers Council, 2011.

