Operating Costs of Sanitizers
Comparing Operating Costs of Sanitizers: Calcium Hypochlorite and Trichlor
Water Usage
Water usage is a big component of running any aquatic facility. As mentioned, cal hypo adds calcium hardness and trichlor adds cyanuric acid. Neither CH or CYA are destroyed by chlorine, nor do they evaporate. Draining and replacing the water is the only practical way to decrease concentrations. Splash out and back washing the filter will reduce these concentrations, however estimating water consumption rates of these activities is difficult and can change from pool to pool, so water usage from these activates were left out of this paper’s estimates. Since evaporation does not remove either chemical, water consumption from evaporation was not factored directly into costs
- Calcium hardness (CH) plays a vital role in water chemistry, helping to protect plaster, concrete and metal objects from corrosion caused by aggressive water. A minimum of 150 ppm CH is required for pool water (ANSI/APSP-11 15), with 200 ppm CH recommended for plaster pools (NPC). Between 150 – 1000 ppm CH, the saturation index can be maintained to prevent scale and cloudy water formation (ANSI/APSP-11 15).
- Adding 10 ppm AvCl2 using calcium hypochlorite will add 8 ppm CH (APSP Calcium). For a pool starting at 150 ppm CH, adding 10 ppm AvCl2 daily with calcium hypochlorite would cause the CH in the pool water to reach 1006 ppm in 107 days. When the water reaches 1000 ppm CH, removing and replacing 1 % will drop the CH 8 ppm if the source water is 200 ppm CH. This will counter the CH added by the daily dose of calcium hypochlorite at these concentrations and rates. In 100,000 gallon pool this is equal to 1000 gallons.
- Cyanuric acid (CYA) helps protect the available chlorine from decomposition caused by UV light. A maximum of 100 ppm CYA is recommended by ANSI/APSP-11 2009, and a maximum 90 ppm CYA by the Model Aquatic Health Code (MAHC 211). CYA is unnecessary and not recommended for indoor pools (ANSI/APSP-11 22).
- Adding 10 ppm AvCl2 using trichlor will add 6 ppm CYA (APSP Trichlor). For a pool starting at 30 ppm, adding 10 ppm AvCl2 daily with trichlor would cause the CYA in the pool water to reach 90 ppm in 10 days and 102 ppm in 12 days. At 90 ppm CYA, 6.7% of the water must be removed and replaced to counter this daily addition of trichlor. At 100 ppm CYA, removing and replacing 6.0% of the water will counter the CYA added daily by the use of trichlor. For a 100,000 gallon pool that is 6,700 gallons and 6,000 gallons respectively.
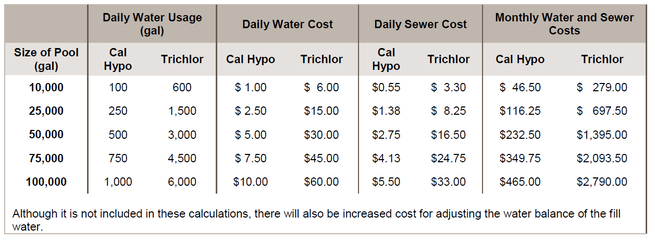
Water and sewage costs are a big opportunity for pool operators to save money. The examples above show that to provide the same amount of available chlorine, it can take 6 times or more water to keep the CYA in a recommended range when using trichlor than the amount of water that is needed to keep the CH in a recommended range when using calcium hypochlorite. Since this water must be drained and refilled, it stands to reason that in locations where sewer costs are based on consumption, not only would the water costs be 6 times higher, but sewer costs would be as well.
Chemical costs
32% muriatic acid – $0.055 per ounce ($7.00 per gal) Trichlor – $2.16 per pound
Sodium carbonate – $1.80 per pound
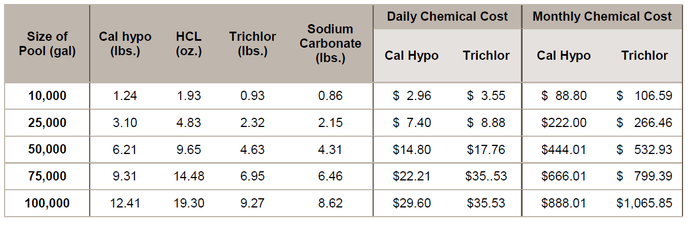
The chemical cost to counter the pH effect of 1 lb. of cal hypo adds $0.09, where the chemical costs to counter the pH effect of 1 lb. of trichlor adds $1.67 per pound. The combined chemical costs per pound of cal hypo would be
$2.39, and per pound of trichlor would be $3.83. With cal hypo at 68% AvCl2, and trichlor at 90% AvCl2, the combined chemical costs per pound of AvCl2 for cal hypo would be
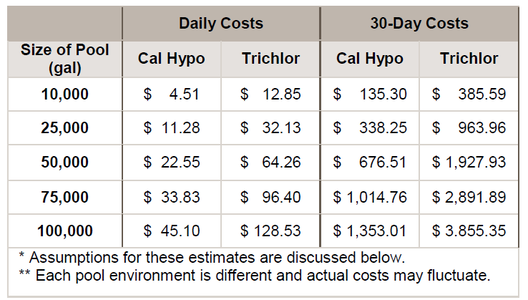
$3.51 and trichlor would be $4.26. With 10 ppm AvCl2 is provided daily, the chart below estimates the daily and monthly costs for several pool sizes:
Estimated Water, Sewer and Chemical Costs
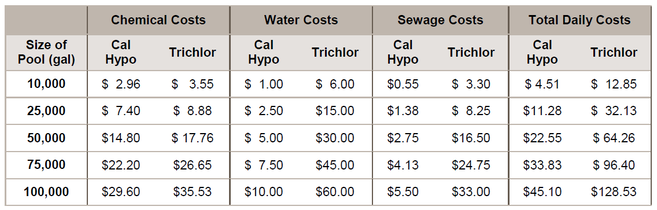
The chart below combines the daily chemical the water and sewage costs that would be charged by Fulton County, Georgia:
References
APSP. “Calcium Hypochlorite.” June 2010. APSP.org. The Association of Pool and Spa Professionals. Fact Sheet. 18 April 2017. <http://www.apsp.org/Portals/0/RWQ%20Fact%20Sheets/Calcium%20Hypochlorite%20- %20July%2029,%202014.pdf>.
“MAHC.” The Model Aquatic Health Code. Atlanta: The Centers For Diseas Control and Prevention, 15 July 2016. 211.
Web. 18 April 2017. .. <https://www.cdc.gov/mahc/pdf/2016-mahc-code-final.pdf>.
NPC. “Start-up Cards.” Vers. 3. 18 April 2017. npconline.org. National Plasterer’s Council. pdf. 18 April 2017.
<https://c.ymcdn.com/sites/npconline.site-ym.com/resource/resmgr/Docs/startup-card-current-rev3_wa.pdf>.
Water & Sewer Rates. n.d. City of Atlanta Department of Watershed Management. Web. 18 April 2017.
<http://www.atlantawatershed.org/customer-service/rates/>.

