Cyanuric Acid (CYA) Buildup
Cyanuric Acid (CYA) Buildup
If you are adding stabilized sanitizer to your pool, it is inevitable that you are also adding cyanuric acid (CYA) to the pool. When stabilized sanitizer is added to the water, the active chlorine is used up, but the CYA remains. Over time, the CYA concentration can build up to unacceptable levels.
CYA can be added to the pool by adding CYA stabilizer to the pool, or by adding the forms of stabilized chlorine, trichloroisocyanuric acid (trichlor) and dichloroisocyanuric acid (dichlor). The pounds of CYA added to a pool for every 100 pounds of stabilized sanitizer are shown in the following table:
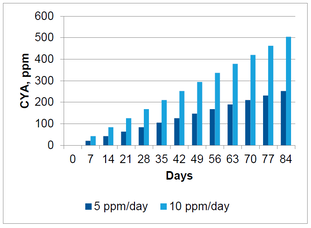
For a pool feeding 5 ppm of AvCl every day using trichlor, the amount of CYA contributed to the pool is 3 ppm per day. The following graph shows how CYA can accumulate over time for a pool receiving 5 ppm and 10 ppm per day of AvCl using trichlor.
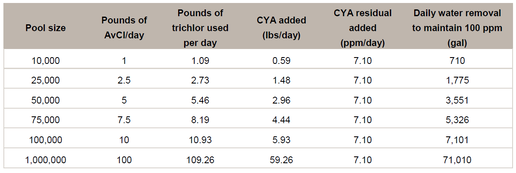
For most pools, the only practical way to remove CYA is to drain part of the pool. For a pool that has 100 ppm of CYA and that is being fed 1 lb/day/10,000 gallons of available chlorine using trichlor, in order to maintain a constant CYA concentration at 100 ppm, 710 gallons of water would need to be drained from the pool each day.
The preferred option is to prevent buildup of CYA by using unstabilized sanitizers. If CYA is needed to stabilize the chlorine residual, it can be added separately in a controller manner.
If you are adding stabilized sanitizer to your pool, it is inevitable that you are also adding CYA to the pool. The reason for this is that CYA is part of the molecular structure of trichloroisocyanuric (trichlor) acid and dichloroisocyanuric acid (dichlor). The following diagram shows the structure for trichlor. As seen in the diagram, trichlor contains three chlorine atoms. Dichlor has a similar structure, except that it has two chlorine atoms.
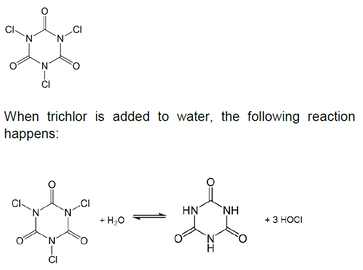
The chlorine atoms come off of the cyanurate ring and form the active form of chlorine in pools, hypochlorous acid (HOCl). As more trichlor is added to the pool, the active chlorine is used up, but the CYA remains. Over time, the CYA concentration can build up to unacceptable levels.
The buildup of CYA will depend on how frequently it is added to, and removed from, the pool.
CYA can be added to the pool by adding CYA stabilizer to the pool, or by adding the forms of stabilized chlorine, trichlor and dichlor. It is easy to calculate how much CYA is added from these products. The follow table shows how the percentage of CYA is calculated from the molecular weight of the trichlor molecule. The total molecular weight for trichlor is 232.41 g/mole. The carbon, nitrogen and oxygen atoms make up the CYA ring and total up to 126.06 g/mole. This value is 54.2% of the total.Similarly, the percentage of CYA can be calculated for dichlor.
The following table shows the percentage of CYA in each of the following chemicals.
This means that for every 100 pounds of trichlor that is added to the pool, 54.2 lb of CYA is added to the pool. If the product you have is not 100% trichlor, or dichlor, then the value can be adjusted by looking at the % ingredients on the label. For instance, for a product that is 99% trichlor, that product contains 54.2 x 0.99 = 53.7% CYA.
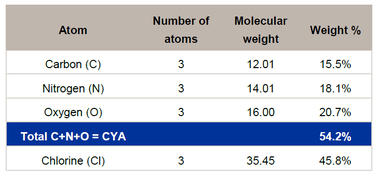
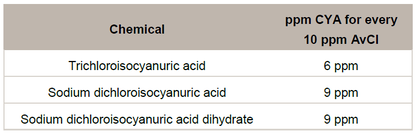
Another way to look at these values is to compare the percentage of CYA to the percentage of available chlorine (%AvCl). The available chlorine of 100% trichlor is 2 x 45.8% (see Table 1 above) = 91.6%. The ratio of 91.6% (%AvCl) to 54.2% (%CYA) is approximately 5:3. So for every 5 ppm of available chlorine added to the pool with trichlor, 3 ppm of CYA is added.
The following table shows the amount, in ppm, of CYA that is added for every 10 ppm of AvCl:
For a pool feeding 5 ppm of AvCl every day using trichlor, the amount of CYA contributed to the pool is 3 ppm per day. The following graph shows how CYA can accumulate over time for a pool receiving 5 ppm and 10 ppm per day of AvCl using trichlor.
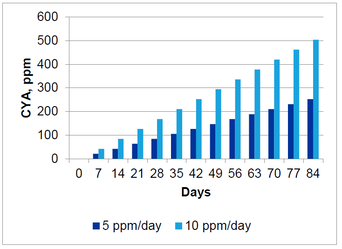
This graph shows that within a three month pool season, the CYA can easily climb to unacceptable levels. The current maximum limit set in the Model Aquatic Health Code is 90 ppm. This level is exceeded within three weeks feeding 10 ppm/day and within 5 weeks feeding 5 ppm/day.
The rate of CYA depletion is much more difficult to predict because it will depend on several factors, some of which cannot be controlled. Since CYA is not destroyed by sunlight or chlorine levels maintained in pools, the only practical way it can be removed is through removal of the water. Water removed from the pool by backwashing filters can be measured and controlled. However, it is more difficult to measure and control the loss of water due to splash out, drag out, and leaking of the pool. Water lost from the pool due to evaporation does not reduce the CYA concentration because CYA does not evaporate with the water. Accordingly, the total water loss from the pool cannot be used to predict the CYA loss from the pool.
The following table was created to calculate how much water would need to be drained from a pool containing 100 ppm of CYA to maintain the 100 ppm of CYA when 1 lb/day/10,000 gallons of AvCl is being fed using trichlor.
The table shows that to maintain a CYA concentration of 100 ppm, 710 gallons would have to be drained from a 10,000 gallon pool every day. Within a two week period, a sum of 10,000 gallons would have been drained from the pool.
Clearly this is not an economically or environmentally preferred option, particularly in drought prone areas with water restrictions.
There are various techniques for removing CYA from pool water, including but not limited to the following techniques; however, there are drawbacks to each of them.
- Melamine precipitation – the reagent used to measure CYA levels in pool water, melamine, is a chemical that binds CYA and forms an insoluble precipitate. Although the complex is insoluble, it does not settle very well and can be difficult to remove from the pool once formed. Colored complexes can also occur that may stain pool surfaces.
- Activated carbon – activated carbon can remove CYA from water, but it is not very efficient. Disposal of the spent carbon can also be an issue if local regulations classify it as a hazardous waste.
- Reverse osmosis – reverse osmosis (“RO”) can be used to remove CYA from pool water. The efficiency of RO processes can vary significantly. If an RO system has 80% recovery, this means that for every 10,000 gallons of water that is treated, ~ 8,000 gallons of clean water will be produced, and ~ 2,000 gallons of concentrated will be waste. Disposal of the RO waste may be regulated by local authorities.
Instead of removing CYA from pools, it is preferred option is to prevent buildup of CYA by using unstabilized sanitizers. If CYA is needed to stabilize the chlorine residual, it can be added separately in a controller manner.
References
Wojtowicz, J.A., 2001, Factors affecting the cyanuric acid concentrations in swimming pools, Journal of the Swimming Pool and Spa Industry, 4(2), 17-22.
US Patent 3,835,136 Hirdler, L.C., Schiessl, H.W., Doonan, D.F., Recovery of chlorine and cyanuric acid values from polychloroisocyanuric acid and salts thereof, 9-10-74 (activated carbon)
US Patent 4,793,935 Stillman, N. W., Method of removing cyanuric acid from bather water, 12-27-88 (melamine).
Archives
400-200-00056 Sandel, B.B., 5/7/92, Cyanuric acid removal from swimming pools
400-200-00045 Green, D.L., 8/17/87, Cyanuric acid adsorption by GAC
400-200-00047 Knoop, D.F., 5/21/92, Cyanuric acid remover (melamine)
400-200-00043 Blanchette, D.W., 11/5/87, Evaluation of Melatrine (1,3,5-trizaine-2,4,6-amine) cyanuric acid remover from Applied Biochemists, Inc.
400-200-00092 Liao, H.P., 1/11/80, South Charleston CDB plant pollution abatement: Part II, Removal of cyanuric acid from waste streams by melamine precipitation.
400-200-00090 Castrantas, W.H. FMC Corporation, 3/21/73, Effect of superchlorination with sodium hypochlorite on cyanuric acid removal
400-200-00060 Kraitchman, L.A., 3/31/82, Carbon adsorption of cyanuric acid in the treated Trichloroisocyanuric acid filtrate.

